Which of the Following Atoms Has the Least Metallic Character
As we move along a period radius decreases and as we move down the group radius increases so F have smallest radius. 2What type of a reaction occurs when Calcium metal reacts with fluorine gas.

Metallic Character Trend On The Periodic Table
Beryllium is an Alkaline Earth Metal.

. Which of the following atoms has the least metallic character. D gas evolution. Na K Rb 38.
Quiz4 Question 1 of 12 00 40 Points Which of the following atoms has the least metallic character. From phosphorus to sulphur to chlorine non-metallic character gradually increases chlorine being the most non-metallic in behaviour. YOU MIGHT ALSO LIKE.
Which of the following atoms has the least metallic character. Li Na OK Cs Rb O O. Chem test 2 sem 2.
Metallic character is displayed by metals which are all on the left-hand side of the periodic table. Which of the following has lowest metallic radius. Which element has the most non-metallic character among oxygen nitrogen fluorine and carbon.
C Li Fr Na. What element in period 3 has the most metallic character. Thus B e has least metallic character.
The least metallic for period 3 is Si. Li Na K Rb. The order of non metallic character among halogens is.
Since the noble gases are a special group because of their lack of reactivity the element fluorine is the most reactive nonmetal. O a Rb O b. Si P S d.
In the 18 or zero group argon does not exhibit either metallic or non-metallic character. The exception is hydrogen which is a nonmetal under ordinary conditions. Which of the following atoms has the least metallic character.
Boron is a Metalloid and is the least metallic. Which of the following atoms has the greatest metallic character. As the elements of Group 17 VIIA are considered in order of increasing atomic number the nonmetallic character of each successive element A.
Which of the following atoms have the following electron configuration 1s2 2s2 2p6 3s1 Na Na Ne Cl. Fluorine gas reacts explosively with many other elements and compounds and is considered to be one of the most dangerous known substances. 3How many moles of Cu are in Cu atoms.
This is due to the fact that the increase in atomic radius decreases attraction between the positive nucleus and the negative electrons causing the electrons to be held more loosely. Which of the following atoms has the least metallic character. Up to 24 cash back As an example the metallic character of Beryillium 4 would not be as great as the metallic character of Barium 56.
How are the patterns seen in the periodic table for ionization energy and electron. B N C b. Cs O eli.
Which form of electromagnetic radiation has photons with the highest energy. It is in a way that as we move across the periodic table tendency to accept electrons increases which. F Cl S c.
All of these are non-metals but the most non-metallic is Fluorine. F Cl Br I As you see fluorine is the most reactive non metal whereas iodine is the least reactive one. Which of the following atoms has the least metallic character.
We found cesium strontium aluminum sulfur chlorine and fluorine on the periodic table. There is no such clear division between metallic and non metallic character. All given elements are of group IIA and B e is top most in that group.
Metallic character is a characteristic property of metals which are all on the left-hand side of the periodic table. 1Which of the following atoms has the least metallic character. Even hydrogen behaves as a metal when its a liquid or solid but you should consider it nonmetallic for most purposes.
Which of the following elements have the least metallic character. Which is the least metallic alkali metal. P S Se e.
It is not found in nature as a free element. Lithium is an Alkali Metal. Correct option is A On moving down the group from Carbon to Tin the metallic character increases.
The metallic character of the elements increases down the group. Hence option D is correct. Silicon is midway between metals and non-metals.
Hence Be will have the least metallic character in the given options. Which of the following atoms is the smallest. The most metallic is Al.
Cesium is the farthest left and the lowest while fluorine is the farthest right and the highest so we know they have the highest metallic character and the lowest metallic character respectively. B acid-base neutralization. O a Rb O b.
Which of the following atoms has the least metallic character. MCAT Mometrix Comprehensive Guide. C Question 2 of 12 40 40 Points What is the element in which at least one electron is in the d-orbital.
Francium is extremely rare and is radioactive with the longest half-life at 22 min so there is no empirical evidence that francium is the most metallic element. The alkali metals in group 1 are the most active metals and cesium is the last element in the group for which we have experimental data. Consider the electron configuration of the ion to determine which ion shown below has an.
The list that correctly indicates the order of metallic character is _____. Hydrogen is an exception which is a nonmetal under ordinary conditions but even hydrogen behaves as a metal. Therefore option A is the correct answer.
Which of the following atoms has the greatest metallic character. Which element has the highest electron affinity. ANa Incorrect BCs CLi DRb EK Answer Key.

How Do You Arrange Elements In Order Of Increasing Metallic Character
Metallic Nature Video Periodic Table Khan Academy

Which Is More Metallic Potassium Or Aluminium Quora

1 Chapter 3a Atoms And Elements 2 Chapter Outline Elements And Symbols Elements And Symbols Periodic Table Of The Elements Periodic Table Of The Ppt Download

Chem Chapter 8 Flashcards Quizlet
Which Element Has The Most Non Metallic Character Among Oxygen Nitrogen Fluorine And Carbon Quora

What Are The Least Metallic Elements Quora
Which Is More Metallic Na Or K Quora
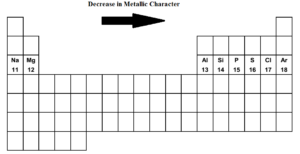
Metallic Character Of Third Row Elements Meaning Trend Factors Affecting

Metallic And Nonmetallic Character Chemistry For Non Majors

Chapter 9 Electrons In Atoms And The Periodic Table Ppt Video Online Download
What Are The Metallic And Non Metallic Characters In Group 4 Elements Quora

Metallic Character Trend On The Periodic Table

Alkali Metal Definition Properties Facts Britannica
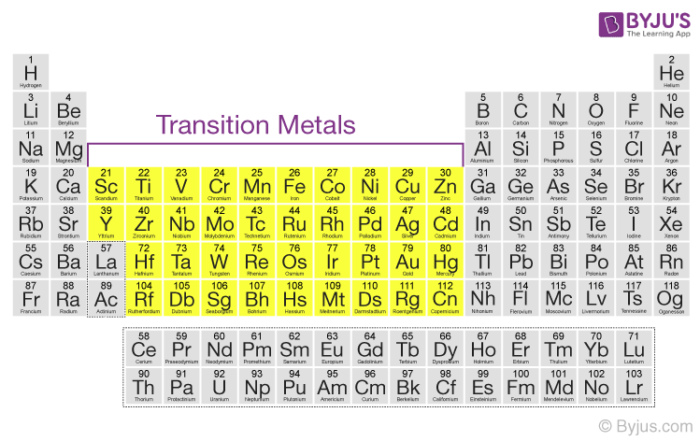
Metallic Character Of Transition Metals Transition Element
Metallic Character The Periodic Table Of Elements

1 Chapter 11 The Chemical Elements Dr Babar Ali Ppt Download


Comments
Post a Comment